Electrochemistry
Electrochemistry
Diamond also has very attractive material properties for electrochemical systems. Diamond is typically boron doped to improve the conductivity in such applications. The advantages of boron doped diamond (BDD) include the widest solvent window of all electrode materials, low background and capacitive currents, reduced fouling compared to conventional electrodes, and the ability to withstand extreme potentials, corrosive, and high temperature/pressure environments. For these reason, BDD has gained popularity for electrochemical applications in the fields of electroanalysis, sensor technology, electrosynthesis, and water treatment. Siuzdak et al. have utilized diamond electrodes for the detection of the influenza virus. He et al. explored the use of BDD electrode for the oxidation of organic pollutants to treat wastewater and remove toxins from drinking water. Xu et al. employed diamond to reduce CO2 byproducts resulting from fossil fuel combustion into formic acid and hydrogen.
(Effect of varying the boron content in diamond shown by cyclic voltammetry in 0.1M sulfuric acid.)
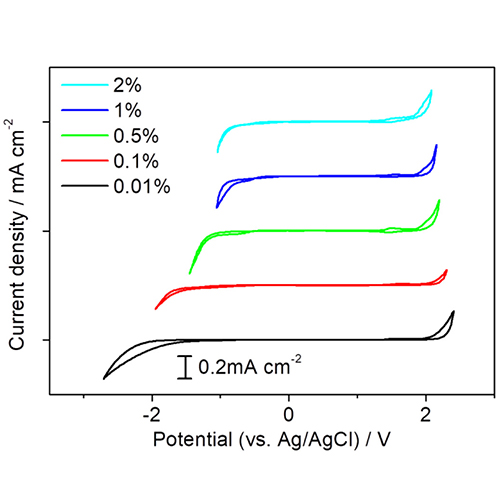
Diamond fabrication parameters has a distinct impact on material properties including dopant density, non-diamond-carbon (NDC) content, grain morphology, and surface chemistry. These features can significantly influence the way the diamond electrode interacts with the electrolyte in electrochemical systems. Boron content effects the electrical conductivity of diamond by acting as an acceptor, attracting electrons from neighboring bonds, creating a pathway for electrons to travel. With a high enough doping concentration, > 1020 atoms cm-3, BDD shows metal-like conductivity. According to Macpherson, metal-like conduction in BDD exhibits a resistivity of <10 mΩ cm. Increasing the dopant concentration can lead to higher capacitance and the likelihood of NDC. Thus, an optimal doping concentration exists which gives the high conductivity without significantly increasing NDC content. Hutton et al. found the optimum concentration to be ~3 x 1020 B atoms cm-3. Increased NDC content results in a more electrocatalytically active electrode which reduces the solvent window and increases the surface’s susceptibility to fouling. The changing solvent window of BDD due to changes in boron content are seen in the figure below where an increase in boron content results in a reduction of the solvent window. An increase in boron content results in a lower peak-to-peak potential separation which indications higher electrochemical reactivity.
Surface termination of the BDD electrode has a strong influence on electron transfer kinetics and wetting properties. Surface termination of BDD electrodes has been studied by many groups; however, it remains a source of contention. To date, a standard methodology does not exist for the determination of the surface functionality of diamond. This is due to several factors including the difficulty in determining the presence of hydrogen termination. Hydrogen surface termination causes semiconducting BDD to behave metal-like due to increased surface conductivity. This behavior can make characterizing BDD difficult as a semi-conductive material can appear to be metal-like until the surface termination changes. The difficulties present in characterizing surface groups on BDD additionally influence the interpretation of results often creating confusing or contradictory findings amongst groups studying similar phenomena.